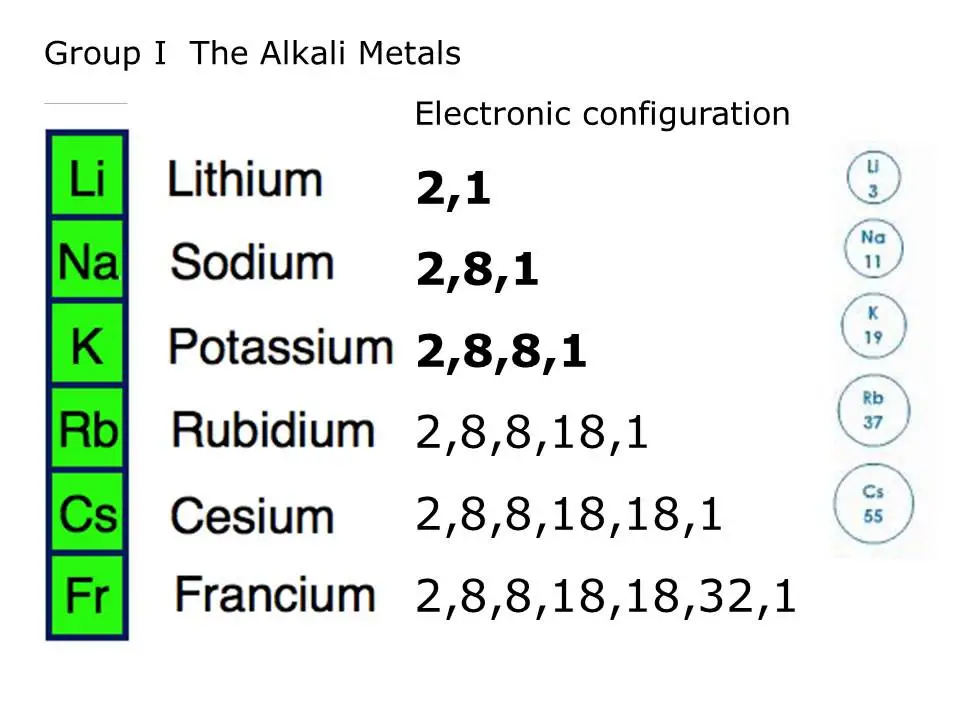
Alkali Metals Boiling Point . Due to this reason, alkali metals. alkali metals are the six different chemical elements found in the first column of the periodic table: Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). The boiling points of the. alkali metals have weak metallic bonding due to presence of only a single valence electron. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). the group 1 elements are all soft, reactive metals with low melting points. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). They react with water to produce an alkaline metal hydroxide solution and hydrogen.
from www.online-sciences.com
in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). the group 1 elements are all soft, reactive metals with low melting points. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). The boiling points of the. Due to this reason, alkali metals. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). alkali metals are the six different chemical elements found in the first column of the periodic table: They react with water to produce an alkaline metal hydroxide solution and hydrogen. alkali metals have weak metallic bonding due to presence of only a single valence electron.
Elements of sblock, Properties of the first group elements 1A (Alkali metals) in the periodic
Alkali Metals Boiling Point The boiling points of the. alkali metals are the six different chemical elements found in the first column of the periodic table: The boiling points of the. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. Due to this reason, alkali metals. They react with water to produce an alkaline metal hydroxide solution and hydrogen. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). the group 1 elements are all soft, reactive metals with low melting points. alkali metals have weak metallic bonding due to presence of only a single valence electron.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Alkali Metals Boiling Point alkali metals have weak metallic bonding due to presence of only a single valence electron. in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). the group 1 elements are all soft, reactive metals with low melting points. alkali metals are the six different chemical elements found in the first. Alkali Metals Boiling Point.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends & Uses Alkali Metals Boiling Point The boiling points of the. Due to this reason, alkali metals. in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). alkali metals are the six different chemical elements found in the first column of the periodic table: cesium is the most volatile of the alkali metals, with a boiling point. Alkali Metals Boiling Point.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Alkali Metals Boiling Point in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). alkali metals are the six different chemical elements found in the first column of the periodic table: alkali metals have weak metallic bonding due to presence of only a single valence electron. Due to this reason, alkali metals. They react with. Alkali Metals Boiling Point.
From studylib.net
1819Alkaline Earth Metals Alkali Metals Boiling Point alkali metals are the six different chemical elements found in the first column of the periodic table: the group 1 elements are all soft, reactive metals with low melting points. Due to this reason, alkali metals. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). in this work, we compiled, evaluated, and selected. Alkali Metals Boiling Point.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Alkali Metals Boiling Point alkali metals are the six different chemical elements found in the first column of the periodic table: The boiling points of the. alkali metals have weak metallic bonding due to presence of only a single valence electron. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). as a result the metallic bonds which. Alkali Metals Boiling Point.
From socratic.org
What bonding types affect the melting and boiling points of Alkaline Earth Metals? Socratic Alkali Metals Boiling Point They react with water to produce an alkaline metal hydroxide solution and hydrogen. the group 1 elements are all soft, reactive metals with low melting points. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. alkali metals are the six different chemical elements found. Alkali Metals Boiling Point.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals Alkali Metals Boiling Point alkali metals are the six different chemical elements found in the first column of the periodic table: the group 1 elements are all soft, reactive metals with low melting points. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). in this work, we compiled, evaluated, and selected recommended values for the normal melting. Alkali Metals Boiling Point.
From byjus.com
Order of boiling point and density for alkali metals Explain Alkali Metals Boiling Point cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). The boiling points of the. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. in this work, we compiled, evaluated, and selected recommended values for the. Alkali Metals Boiling Point.
From selfstudypoint.in
Group 1 Elements Alkali Metals Alkali Metals Boiling Point alkali metals are the six different chemical elements found in the first column of the periodic table: Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). Due to this reason, alkali metals. alkali metals have weak metallic bonding due to presence of only a single valence electron. They react with water to produce an. Alkali Metals Boiling Point.
From www.youtube.com
Melting and boiling point trend among alkali metals S block elements Group 1A elements YouTube Alkali Metals Boiling Point Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). alkali metals have weak metallic bonding due to presence of only a single valence electron. alkali metals are the six different chemical elements found in the first column of the periodic table: The boiling points of the. the group 1 elements are all soft,. Alkali Metals Boiling Point.
From www.youtube.com
Trick To Remember Melting And Boiling Point Of sblock ElementsI Alkali MetalsIAlkaline Earth Alkali Metals Boiling Point alkali metals have weak metallic bonding due to presence of only a single valence electron. the group 1 elements are all soft, reactive metals with low melting points. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). alkali metals are the six different chemical elements found in. Alkali Metals Boiling Point.
From flowvella.com
Alkali Metals Presentation Screen 7 on FlowVella Presentation Software for Mac iPad and iPhone Alkali Metals Boiling Point Due to this reason, alkali metals. the group 1 elements are all soft, reactive metals with low melting points. alkali metals are the six different chemical elements found in the first column of the periodic table: in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). cesium is the most. Alkali Metals Boiling Point.
From www.youtube.com
Boiling point and melting point of alkali metals............. (increase / decrease) withincr Alkali Metals Boiling Point the group 1 elements are all soft, reactive metals with low melting points. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). alkali metals have weak metallic. Alkali Metals Boiling Point.
From www.chegg.com
Solved The graphs below show the melting and boiling points Alkali Metals Boiling Point Due to this reason, alkali metals. They react with water to produce an alkaline metal hydroxide solution and hydrogen. alkali metals have weak metallic bonding due to presence of only a single valence electron. in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). Lithium (li), sodium (na), potassium (k), rubidium (rb),. Alkali Metals Boiling Point.
From www.youtube.com
Alkali metals trends, melting and boiling points, uses and characteristics (Part 1) YouTube Alkali Metals Boiling Point Due to this reason, alkali metals. as a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and. Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). The boiling points of the. alkali metals are the six different chemical elements found in the first. Alkali Metals Boiling Point.
From www.youtube.com
Why do alkali metals have low melting and boiling points ? YouTube Alkali Metals Boiling Point the group 1 elements are all soft, reactive metals with low melting points. alkali metals are the six different chemical elements found in the first column of the periodic table: Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). as a result the metallic bonds which hold solid and liquid alkali metals together. Alkali Metals Boiling Point.
From askfilo.com
Maximum boiling point among the alkali metal halide is of Filo Alkali Metals Boiling Point Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). They react with water to produce an alkaline metal hydroxide solution and hydrogen. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). as a result the metallic bonds which hold solid and liquid alkali metals together. Alkali Metals Boiling Point.
From www.youtube.com
Electronegativity & Melting & Boiling Points of Alkali Metals Gp (1A) (L31) 2nd year Chem Alkali Metals Boiling Point in this work, we compiled, evaluated, and selected recommended values for the normal melting points (tm). Due to this reason, alkali metals. cesium is the most volatile of the alkali metals, with a boiling point of 671 °c (1,240 °f). alkali metals are the six different chemical elements found in the first column of the periodic table:. Alkali Metals Boiling Point.